The specific details of the allocation and rollout of Chinese-donated Sinovac Covid-19 vaccines are still under evaluation pending the recommendation of the National Immunization Technical Advisory Group and approval of the Inter-Agency Task Force for the Management of Emerging Infectious Diseases.
In a statement Thursday, the Department of Health and the National Task Force Against Covid-19 made the clarification, after Malacañang announced that the first batch of Sinovac vaccines, totaling 600,000 doses, will arrive on Feb. 28.
They were also reacting to some reports saying the national vaccination program using the donated vaccines would begin on March 1.
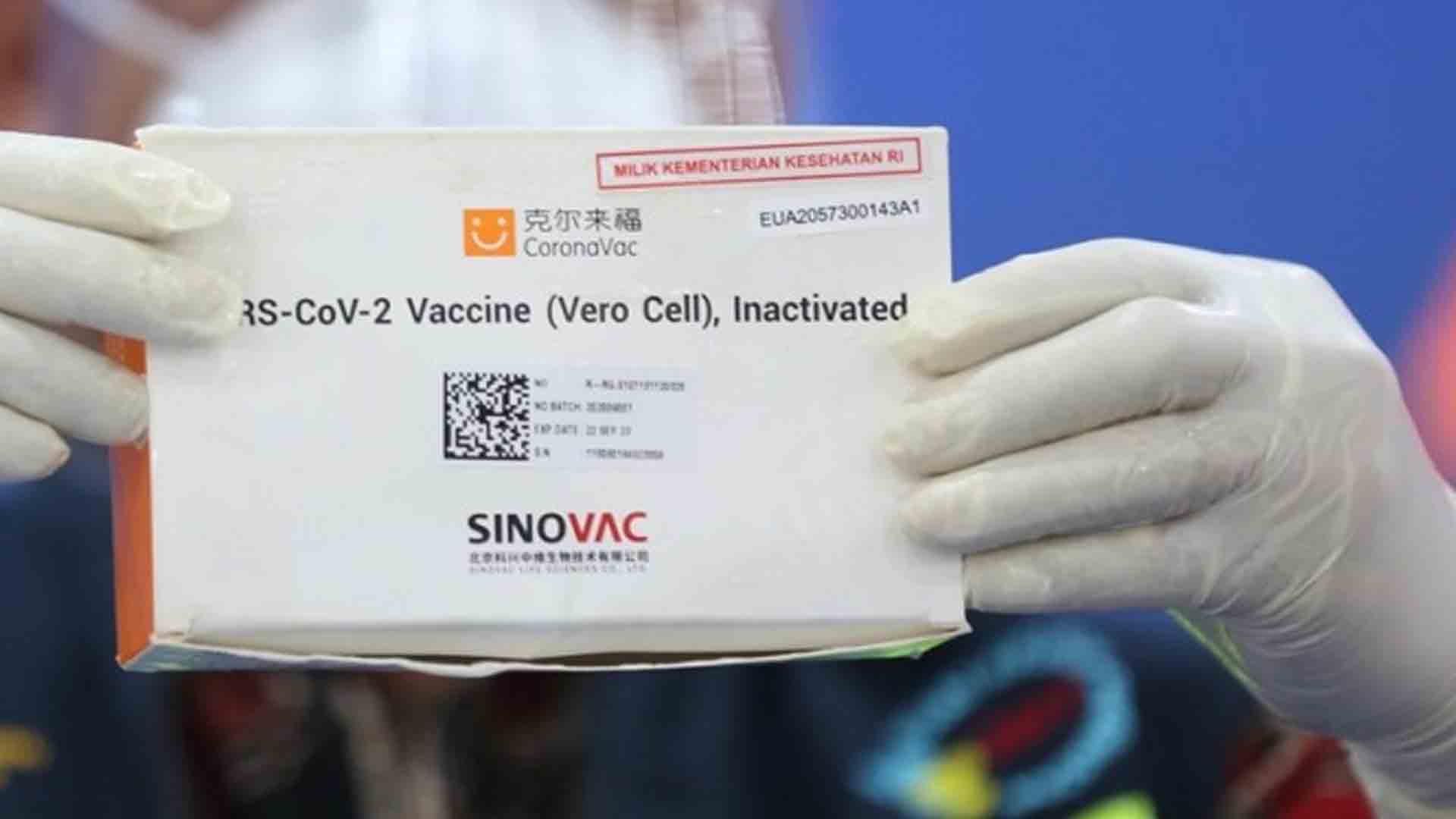
Sinovac’s vaccine, also known as the Coronavac, is the third Covid-19 vaccine to be granted emergency use authorization by the Philippines’ Food and Drug Administration.
AstraZeneca and Pfizer-BioNTech’s Covid-19 vaccines received their EUAs last month.
Citing results of clinical trials, health experts noted that the Chinese vaccine has an efficacy rate of 65.3 as seen in Indonesia and 91.2 efficacy rate in Turkey.
It has a lower efficacy rate of 50.4 percent when used on healthcare workers exposed to Covid-19 so it is not recommended for use to this group.
The vaccines produced by Chinese biopharmaceutical firm Sinovac Biotech Ltd. will be the first to arrive in the country.
Government officials will personally witness the arrival of the vaccines at the Villamor Air Base in Pasay City.
However, details of the event are still being finalized in coordination with the Embassy of the People’s Republic of China. (PNA)